Chem 121 Chapter 5: Introduction to Redox Chemistry
Solved Provide the products of the following reactions. | Chegg.com
Chemical Equation The reaction between zinc and sulfur can be shown in what is called a chemical equation . In words, we could write the reaction as: zinc + sulfur → zinc sulfide The more convenient way to express a chemical reaction is to use the symbols and formulas of the substances involved: Zn + S → ZnS

Source Image: everydayhealth.com
Download Image
You can react to a Pin in different ways: Reacting to a Pin shares your thoughts about it, but does not save the Pin. If you want to see the Pin later, make sure to save it to a board . You can also click on the number of reactions on a Pin and see who reacted to it. Log in to your Pinterest account Click into the Pin you want to react to

Source Image: sarthaks.com
Download Image
Pomegranates 101: A Complete Guide Science > Biology library > Chemistry of life > Chemical bonds and reactions Chemical reactions Google Classroom Chemical reactions and how they break and form bonds between atoms. Balanced reactions, reversibility, and equlibrium. Introduction
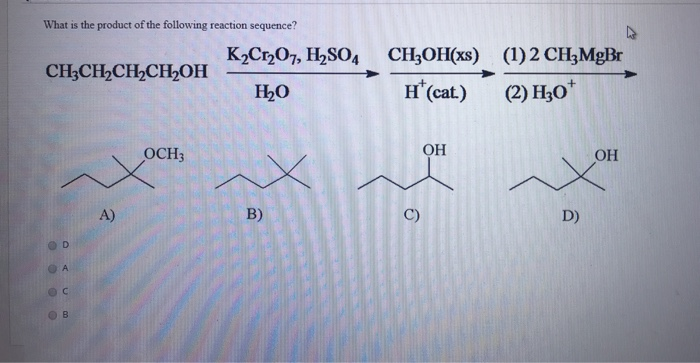
Source Image: chegg.com
Download Image
What Are The Products Of The Following Reactions
Science > Biology library > Chemistry of life > Chemical bonds and reactions Chemical reactions Google Classroom Chemical reactions and how they break and form bonds between atoms. Balanced reactions, reversibility, and equlibrium. Introduction The products of the reaction in the burning of methane as well as other fuels are carbon dioxide and water. The word equation for this reaction is: Figure 11.2
Solved What is the product of the following reaction | Chegg.com
A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product. There is a standard way of writing chemical equations. The reactants are all written on the left-hand side of the equation, with the products on the ‘Natural’ products and skin irritation: How to avoid allergies

Source Image: today.com
Download Image
SOLVED: Predict the product or products of each of the following reactions: (2 PTS EACH) CH3CH2OH C2H5 HCOOH HBr H-C≡C-CH3 + H2 A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product. There is a standard way of writing chemical equations. The reactants are all written on the left-hand side of the equation, with the products on the
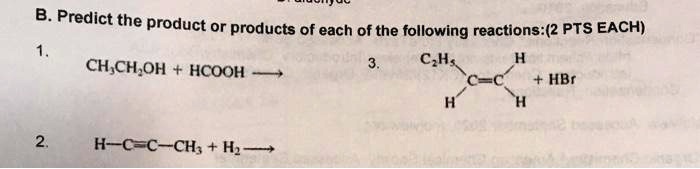
Source Image: numerade.com
Download Image
Solved Provide the products of the following reactions. | Chegg.com Chem 121 Chapter 5: Introduction to Redox Chemistry
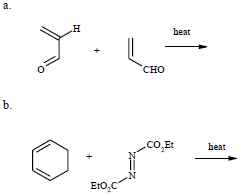
Source Image: chegg.com
Download Image
Pomegranates 101: A Complete Guide You can react to a Pin in different ways: Reacting to a Pin shares your thoughts about it, but does not save the Pin. If you want to see the Pin later, make sure to save it to a board . You can also click on the number of reactions on a Pin and see who reacted to it. Log in to your Pinterest account Click into the Pin you want to react to

Source Image: everydayhealth.com
Download Image
Draw the major product of the reaction sequence show below. | Homework.Study.com Sep 22, 20222 HgO (s) → O 2 (g) + 2 Hg (l) 2 KClO 3 (s) → 3 O 2 (g) + 2 KCl (s) The potential products in double-replacement reactions are simple to predict; the anions and cations simply exchange. Remember, however, that one of the products must precipitate, otherwise no chemical reaction has occurred. For the reaction between lead (II) nitrate and

Source Image: homework.study.com
Download Image
Cleaning Products You Should Never Mix – Cleaning Tips Science > Biology library > Chemistry of life > Chemical bonds and reactions Chemical reactions Google Classroom Chemical reactions and how they break and form bonds between atoms. Balanced reactions, reversibility, and equlibrium. Introduction

Source Image: goodhousekeeping.com
Download Image
Purex Sta Flo Liquid Starch, Great for Crafts, Concentrated, 64 Ounce – Walmart.com The products of the reaction in the burning of methane as well as other fuels are carbon dioxide and water. The word equation for this reaction is: Figure 11.2

Source Image: walmart.com
Download Image
SOLVED: Predict the product or products of each of the following reactions: (2 PTS EACH) CH3CH2OH C2H5 HCOOH HBr H-C≡C-CH3 + H2
Purex Sta Flo Liquid Starch, Great for Crafts, Concentrated, 64 Ounce – Walmart.com Chemical Equation The reaction between zinc and sulfur can be shown in what is called a chemical equation . In words, we could write the reaction as: zinc + sulfur → zinc sulfide The more convenient way to express a chemical reaction is to use the symbols and formulas of the substances involved: Zn + S → ZnS
Pomegranates 101: A Complete Guide Cleaning Products You Should Never Mix – Cleaning Tips Sep 22, 20222 HgO (s) → O 2 (g) + 2 Hg (l) 2 KClO 3 (s) → 3 O 2 (g) + 2 KCl (s) The potential products in double-replacement reactions are simple to predict; the anions and cations simply exchange. Remember, however, that one of the products must precipitate, otherwise no chemical reaction has occurred. For the reaction between lead (II) nitrate and